What We Do
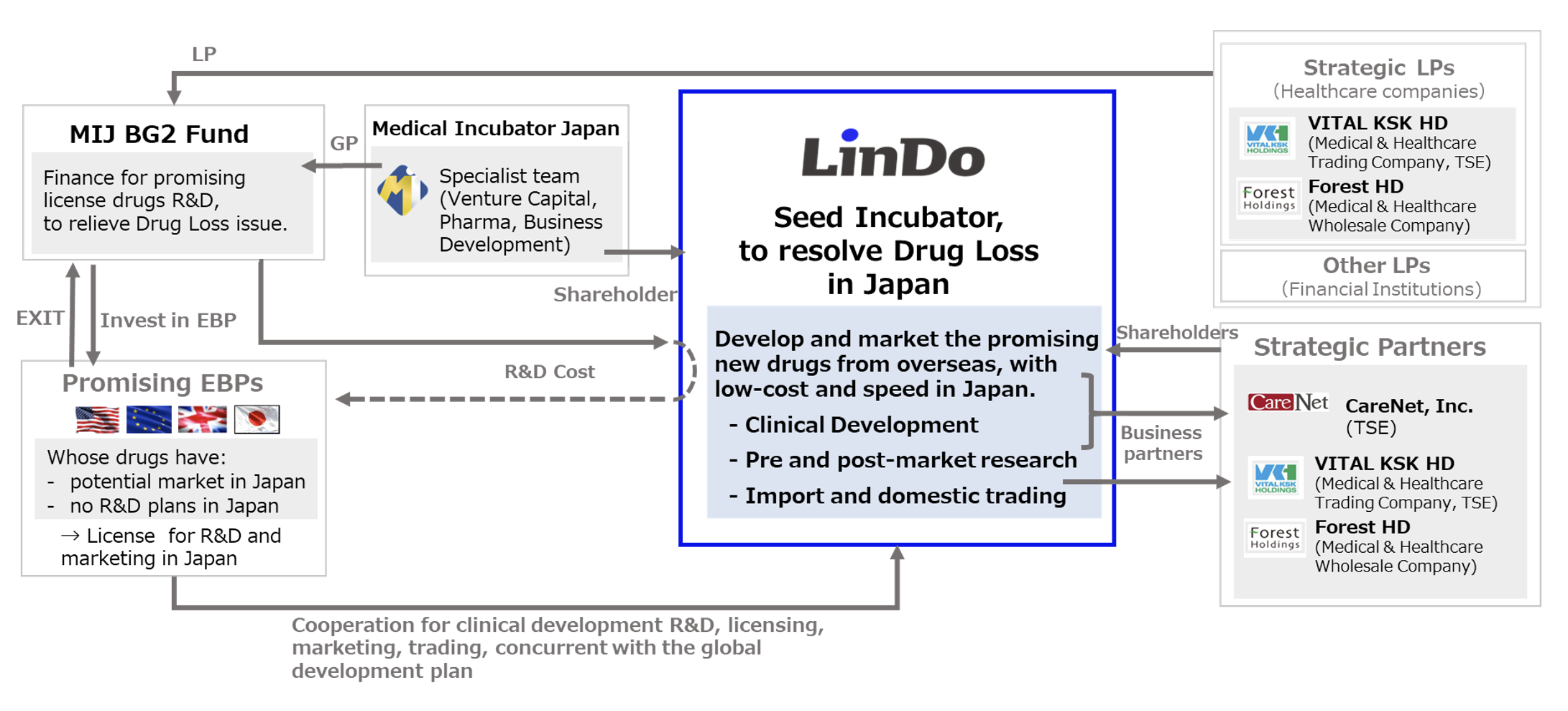
Seed Incubator, LinDo K.K.
LinDo is what is known as an incubator, a company that takes the “seeds” of innovative new drugs, grows those seeds, and launches those new drugs onto the market. Its goal is to create an environment that offers the same level of choice of therapeutic drugs as the US and Europe to the frontlines of treatment of rare disorders and intractable diseases in Japan.
In today’s pharmaceutical market, the central focus of drug development is on diseases for which there is a low degree of treatment satisfaction. Drugs aimed at treating rare disorders, intractable diseases, and rare cancers account for the majority of new drug development around the world. Over 70% of the new drugs being created are coming not from the existing pharmaceutical companies, but in emerging biopharma (EBP) companies, which have an extremely high level of drug discovery technology.
Among the new drugs developed by these EBPs, those that are suitable for large-scale manufacture are licensed by the large pharmaceutical companies, which conduct clinical trials in Japan and eventually launch them on the Japanese market. While the EBPs obtain financing to continue their clinical development of the remaining drugs that are not taken up by Big Pharma, it is not easy for them to conduct clinical development in Japan as well as in Europe and the US.
In the pediatric domain, in particular, Japan lags far behind world standards in the development of new drugs
There are three main reasons for this
Limited development funds
In general, EBPs’ first priority is to obtain approval in Europe and the US. As such, they do not have the financial wherewithal to undertake clinical development in Japan.
Less Information of the Japanese market
For EBPs, it is not easy to understand the Japanese market, including its structure and potential, and of how to handle Japan’s approval system.
Development network
The challenge of corporate networking and partnering for conducting development in Japan remains unresolved.
The compounding of these three reasons has led to the social issue of “drug loss,” in which many new drugs are not developed or approved in Japan, and it is the great hope of patients and healthcare professionals seeking new treatments that they be developed for the Japanese market.
Using a development scheme created in partnership with Medical Incubator Japan K.K. (MIJ), whose people have a wealth of experience working for pharmaceutical companies, LinDo, as seed incubator, will promote the clinical development of innovative new drugs with potential for treating rare disorders and intractable diseases, with the aim of obtaining approval for those drugs in Japan.