TO ALL EMERGING BIOPHARMA (EBP)
For Biopharma

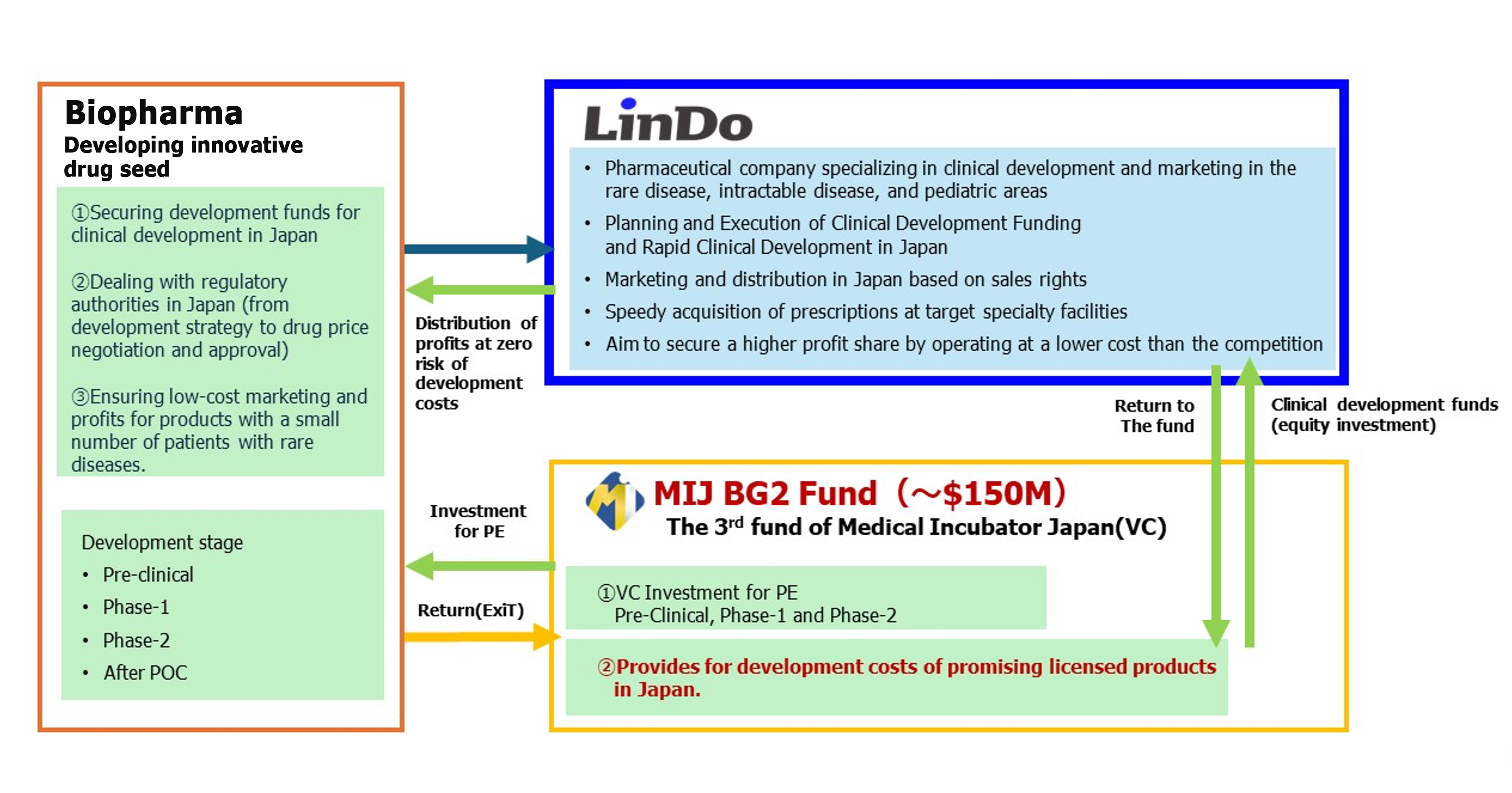
LinDo K.K. is a pharmaceuticals company that specializes in licensing new therapeutic drugs, including those for rare disorders and intractable diseases, being developed by emerging biopharma (EBP) companies in such as US and Europe, and developing those drugs, obtaining approval for them, and marketing them in Japan.
LinDo is a specialty pharma company, headquartered in Tokyo, and operates with a new business model that integrates a pharmaceutical company with a fund.
We are a company that prioritizes efficiency and accuracy and our staff have a wealth of experience guiding a large number of products to success during many years working in global pharmaceutical business in areas from development to marketing, sales, and distribution.
The LinDo shareholders include specialty CRO supporting clinical development for rare disorders and intractable diseases, and companies with networks of the medical specialists who are treating rare disorders and intractable diseases in Japan. This enables us to offer a one-stop service for everything from clinical development to the activities after the launch of commercial sales.
We have thoroughly refined our practices and trimmed away the personnel costs and wasteful excessive processes that can be found at the existing pharmaceutical companies in Japan. This makes it possible for us to demonstrate high performance that could never be imitated by the major pharmaceutical companies and CRO.
CHALLENGES
We have recently entered an era where the various EBP companies such as yourselves are leading the development of new drugs with new modalities. However, a large number of companies are targeting their focus on improving their corporate value through development and success in the United States and Europe, which form the primary markets. In terms of the independent pharmaceutical market size per country, Japan has the third largest market in the world after the United States and China. In addition, Japan’s universal public health insurance system means that there can be a rapid increase in sales after the launch of a product on the medical pharmaceutical market. Once a product has entered the market, it is possible to secure large profits. However, as a result of the reasons outlined below, 70% of the products from EBP have no plan for clinical development in Japan.
Challenges for EBP in Japan
The high cost and slow development speed of clinical development in Japan compared with that in Europe and the United States
Insufficient understanding of the development, approval, and drug price acquisition processes in Japan
The difficulty of meeting reliable partners in the Japanese market who are highly familiar with the Japanese market
ADVANTAGES
LinDo works based on license contracts with EBP that have no development plan for Japan. We provide complete support for the development costs in Japan and also perform the work to submit applications to the authorities and all the other pharmaceutical manufacturing-related operations from the data collection after launch to the sales and distribution work.
In reality, these EBP do not want to spend time and money on consideration of the Japanese market. LinDo is a company that can provide a solution to this situation and work together with your company to mutually raise the corporate value of both of our companies.
The advantages offered by LinDo
The costs for clinical trials in Japan
Dealing with the regulatory regime in Japan (Japanese)
The proposal and execution of product strategy plans for Japan (clinical development to medical strategy and sales strategy)
Securing of a good relationship with the medical specialists and facilities related to clinical trials and marketing
Medical system that secures safety and data acquisition with global quality
Securing of sales and profit in Japan
METHOD
As a specialty pharma company, LinDo provides a one-stop solution to these problems and will work with you on everything from the development to the sales of your products in Japan and the strengthening of your company brand.
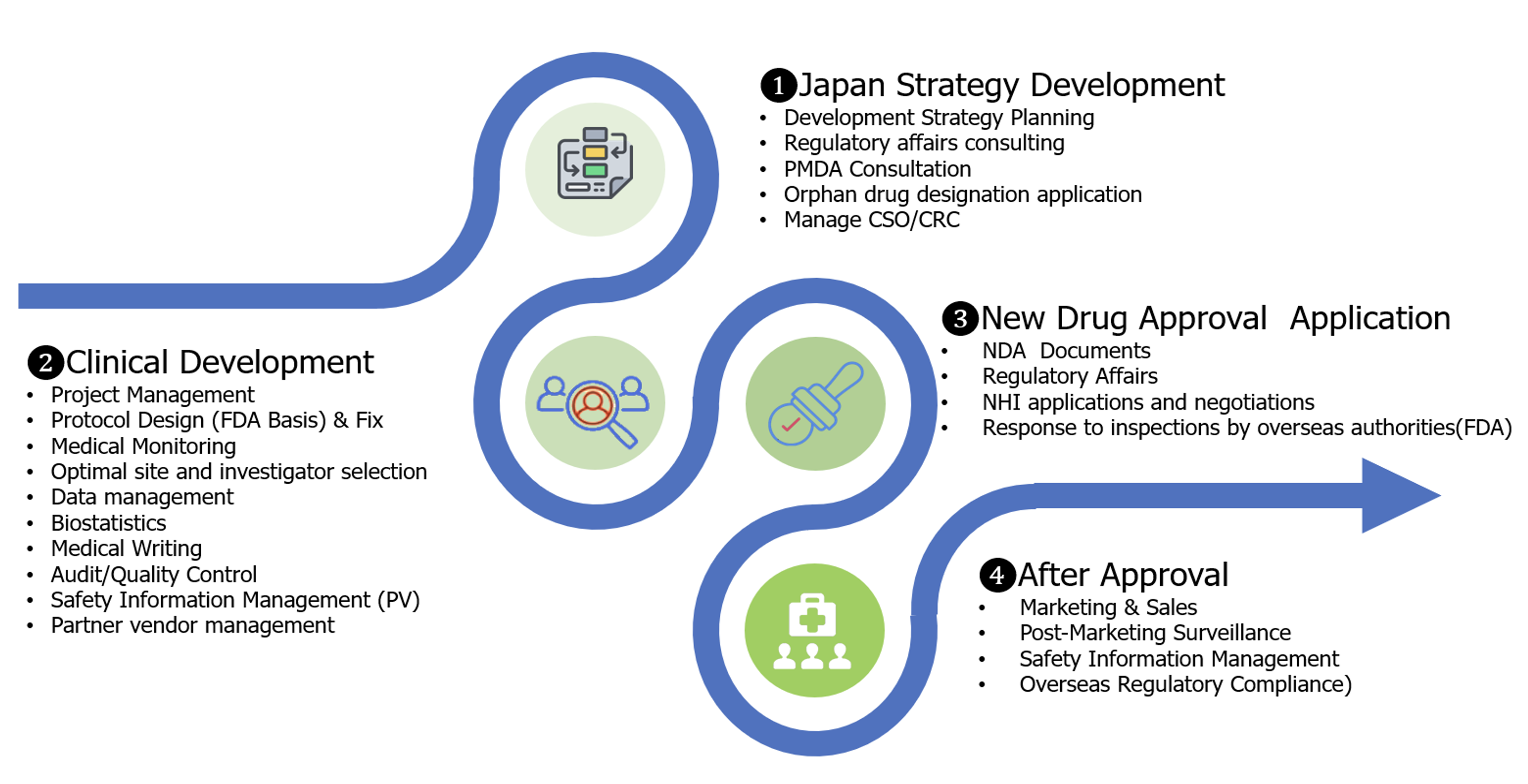
As a specialty pharma company, LinDo provides a one-stop solution to these problems and will work with you on everything from the development to the sales of your products in Japan and the strengthening of your company brand.
Securing the costs for clinical trials in Japan
- When entering the Japanese market, LinDo will obtain the necessary money from the fund for clinical trial costs that may exceed those in the United States and Europe. LinDo will bear those expenses for clinical trial in Japan, which means no risk to EBP. This is based on the fact that LinDo holds the exclusive license for the development and sales within Japan.
The product strategy planning for Japan (clinical development to medical strategy and sales strategy) and execution management
- We have staff members with more than 30 years of experience working in top management positions in drug development departments in Japan and in the Japanese branches of global CRO. This enables us to avoid the problems that companies expanding into Japan tend to experience and to secure the sense of speed that the development companies are aiming for in the Japanese development.
- We work based on the product profile and development strategy of EBP and propose the minimal development plan required for development within Japan.
Dealing with the regulatory regime in Japan
- We follow the development strategy agreed with your company and perform the coordination with the Japanese regulatory authorities (PMDA) and related specialist medical associations.
- Revised drug price acquisition and negotiation
- Acquisition and reporting of safety information
Securing of a good relationship with the medical specialists and facilities related to clinical trials and marketing
- For the selection of the medical specialists and facilities that will enable participation by appropriate patient participants in the clinical trials, we fully utilize our shareholder companies which have databases of doctors involved in rare disorders and intractable diseases in Japan
- In order to maintain the quality of the patient enrollment, we fully utilize the medical personnel of these companies
Medical practice that secures safety and data acquisition with global quality
- We have experience in a large number of the Japan projects of global pivotal studies for foreign IBP products
Securing of sales and profit in Japan
- Based on more accurate targeting of specialists based on the number of patients from the preparatory stage of clinical development, we provide and collect information to specialists and penetrate the market in the most effective and efficient marketing mix.
If you are interested in our services, please contact at info@lindosi.com.